What is Ionic bond?
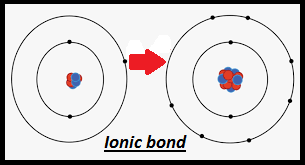
Ionic holding is a sort of synthetic holding that includes the electrostatic fascination between oppositely charged particles, or between two iotas with pointedly various electronegativities, and is the essential connection happening in ionic mixtures. It is one of the principal kinds of holding alongside covalent holding and metallic holding. Particles are iotas (or gatherings of molecules) with an electrostatic charge. Particles that gain electrons make adversely charged particles (called anions). Iotas that lose electrons make emphatically charged particles (called cations). This exchange of electrons is known as electrovalence as opposed to covalence. In the easiest case, the cation is a metal molecule and the anion is a nonmetal particle, however these particles can be of a more mind boggling nature, for example atomic particles like NH+
4 or SO2−
4. In more straightforward words, an ionic bond results from the exchange of electrons from a metal to a non-metal to get a full valence shell for the two iotas.
It is critical to perceive that spotless ionic holding — in which one iota or atom totally moves an electron to another — can't exist: all ionic mixtures have some level of covalent holding, or electron sharing. In this manner, the expression "ionic holding" is given when the ionic person is more prominent than the covalent person - that is, a bond where a huge electronegativity distinction exists between the two iotas, making the holding be more polar (ionic) than in covalent holding where electrons are shared all the more similarly. Bonds with to some extent ionic and to some degree covalent person are called polar covalent bonds.
Ionic mixtures lead power when liquid or in arrangement, regularly not when strong. Ionic mixtures for the most part have a high liquefying point, contingent upon the charge of the particles they comprise of. The higher the charges the more grounded the strong powers and the higher the dissolving point. They likewise will quite often be solvent in water; the more grounded the strong powers, the lower the dissolvability.
Thanks for reading!








No comments:
Post a Comment
If you have any doubt so please let us know.